S-Adenosil-L-homosisteina
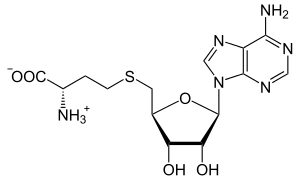
S-Adenosil-L-homosisteina (SAH) ialah molekul pendahulu biosintetik bagi homosisteina.[1] Ia terbentuk melalui penyahmetilan of S-adenosil-L-metionina.[2][3] Adenosilhomosisteinase menukarkan SAH menjadi homosisteina dan adenosina.
| |
| Nama | |
|---|---|
| Nama IUPAC
S-(5′-Deoksiadenos-5′-il)-L-homosisteina
| |
| Nama IUPAC sistematik
Asid (2S)-2-amino-4-({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-il)-3,4-dihidroksioksolan-2-il]metil}sulfanil)butanoik | |
| Nama lain
AdoHcy, 2-S-adenosil-L-homosisteina,
5′-S-(3-Amino-3-karboksipropil)-5′-tioadenosina S-adenosilhomosisteina, SAH | |
| Pengecam | |
Imej model 3D Jmol
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.328 |
| KEGG | |
| MeSH | S-Adenosylhomocysteine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Sifat | |
| C14H20N6O5S | |
| Jisim molar | 384.41 g·mol−1 |
Kecuali jika dinyatakan sebaliknya, data diberikan untuk bahan-bahan dalam keadaan piawainya (pada 25 °C [77 °F], 100 kPa). | |
| | |
| Rujukan kotak info | |
Peranan biologi
suntingDNA metiltransferase direncatkan oleh SAH.[4] Dua kofaktor S-adenosil-L-homosisteina boleh mengikat di tapak aktif DNA metiltransferase 3B lalu menghalang dupleks DNA daripada mengikat terhadapnya sehingga merencatkan pemetilan DNA.[5]
Rujukan
sunting- ^ Finkelstein JD (2000). "Pathways and regulation of homocysteine metabolism in mammals". Seminars in Thrombosis and Hemostasis. 26 (3): 219–225. doi:10.1055/s-2000-8466. PMID 11011839.
- ^ Ribbe MW, Hu Y, Hodgson KO, Hedman B (April 2014). "Biosynthesis of nitrogenase metalloclusters". Chemical Reviews. 114 (8): 4063–4080. doi:10.1021/cr400463x. PMC 3999185. PMID 24328215.
- ^ James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA (August 2002). "Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology". The Journal of Nutrition. 132 (8 Suppl): 2361S–2366S. doi:10.1093/jn/132.8.2361S. PMID 12163693.
- ^ Kumar R, Srivastava R, Singh RK, Surolia A, Rao DN (March 2008). "Activation and inhibition of DNA methyltransferases by S-adenosyl-L-homocysteine analogues". Bioorganic & Medicinal Chemistry. 16 (5): 2276–2285. doi:10.1016/j.bmc.2007.11.075. PMID 18083524.
- ^ Lin CC, Chen YP, Yang WZ, Shen JC, Yuan HS (April 2020). "Structural insights into CpG-specific DNA methylation by human DNA methyltransferase 3B". Nucleic Acids Research. 48 (7): 3949–3961. doi:10.1093/nar/gkaa111. PMC 7144912. PMID 32083663.